A cytotoxic drug bundle is a packed multi-arm linker carrying multiple molecules of a small molecular cytotoxic drug.
The linking arms used in a cytotoxic drug bundle can be optionally designed to contain a cleavable site so that the drug molecules can be cleaved off at the targeted tissues or in the targeted cells. Such a cleavable site is sensitive to an enzyme rich in microenvironment of the targeted tissue or in the endosomes/lysosomes of the targeted cells.
A host of potent cytotoxic drugs such as microtubule inhibitors (monomethyl auristatin E (MMAE) or monomethyl auristatin F (MMAF)), topoisomerase I inhibitors (Deruxtecan (Dxd) or Exatecan (EXT)) and immunomodulatory drugs (IMiDs) such as lenalidomide (Lena), are applicable for preparing cytotoxic drug bundles.
A coupling arm equipped with a functional group for click reaction, such as dibenzocyclooctyne (DBCO), bicyclononyne (BCN), trans-cyclooctene (TCO), or maleimide group, for the conjugation with an antibody molecule.
The aqueous solubility of the drug bundles can be adjusted by varying the compositions of the core and linking and coupling arms.
Typically, a maleimide group and adjacent acidic amino acid residues are installed at the end of coupling arm. The cluster of negative charges potentiate the maleimide group as an electrophile.
Here shows the adjustable features of cytotoxic drug bundles.
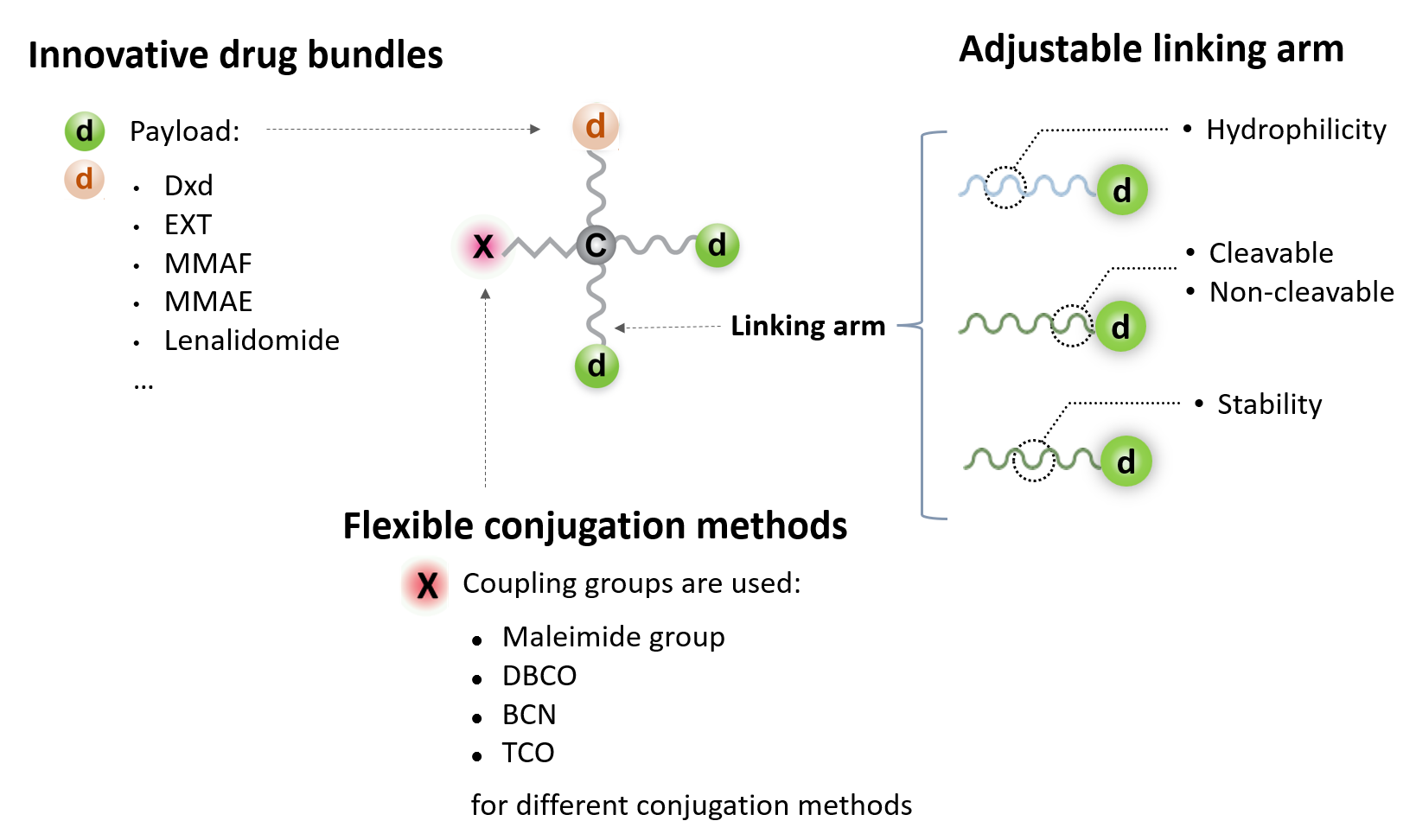
Our cytotoxic drug bundles offer a highly versatile design, enabling the rapid assembly and production of various drug bundles to fit diverse conjugation needs. This facilitates the customized development and scalable manufacturing of ADC therapeutics.
In addition to generating drug bundles carrying a single cytotoxic payload, our platform allows for the rapid and efficient production of dual-cytotoxic drug bundles. This breakthrough overcomes a key technical barrier in ADC development by enabling the conjugation of two distinct payloads, offering new hope for the treatment of drug-resistant and heterogeneous tumors.
We are committed to advancing the application of our drug bundle platform. Regardless of the desired payload combinations, we welcome partners to explore collaboration opportunities and licensing arrangements through various business models.
Enabling the Construction of Two ADC Platforms
When a cytotoxic drug payload carrying a single or dual drug moiety is conjugated to an antibody, an antibody–drug conjugate (ADC) is formed.
Based on distinct antibody configurations, we have further developed two ADC platforms that can be broadly applied to generate next-generation ADC molecules with a high drug-to-antibody ratio (DAR), high purity, and excellent homogeneity.
1. scFv-based ADC
In our optimized design, the antigen-binding fragment (Fab) of the antibody is re-engineered into a single-chain variable fragment (scFv). Consequently, scFv-based ADCs are constructed by conjugating cytotoxic drug payloads to antibody moieties composed of two extended heavy chains associated by interchain disulfide bonds. (For detailed information, please refer to the T-E Meds website: https://www.temeds.com/scfv-based ADC/)
2. CHO-TEM ADC
T-E Meds has established a strategic partnership with CHO Pharma to jointly develop the CHO-TEM ADC platform by leveraging the complementary technologies of both companies. This platform incorporates CHO Pharma’s proprietary glycan modification technology for precise alteration the glycan structure of IgG Fc. Through conjugation of cytotoxic drug payloads to the modified glycan moieties, novel CHO-TEM ADC drug molecules are constructed. (For detailed information, please refer to the T-E Meds website: https://www.temeds.com/CHO-TEM ADC/ )
ADC drug candidates generated from these two ADC platforms successfully demonstrate five key advantages:
- Produce homogenized ADC: Using site-specific conjugation to attach drug bundles to antibodies, enabling the production of high-purity and homogenized ADC drugs.
- Simple and Efficient Process: No need for non-natural amino acids or enzyme catalysis.
- High Drug-to-Antibody Ratio (DAR): Enables the production of ADC drugs with a high DAR, further enhancing therapeutic efficacy.
- Good Solubility and Stability: Through adjustable multi-arm linker designs, the resulting ADC drugs exhibit excellent solubility and stability, improving pharmacokinetic performance and reducing toxicity risks.
- Dual-Payload Capability: Successfully overcomes the technical challenge of dual payloads, enabling ADC drugs to carry two different drugs and providing new opportunities for the treatment of drug-resistant and heterogeneous tumors
We are committed to advancing the application of our drug bundle platform. Regardless of the desired payload combinations, we welcome partners to explore collaboration opportunities and licensing arrangements through various business models.
